Negative Control
Monitor the lower limit fluorescence sensitivity to be sure your instrument is ready for immunophenotyping assays.
BioSure® Negative Control (osmium fixed chicken red blood cells) is a negative standard for assessing the lower limit of fluorescence sensitivity of a flow cytometer. The fixation process quenches cellular fluorescence and results in a particle that has very low autofluorescence. Daily use of this standard in immunophenotyping assays can show that the flow cytometer is capable of detecting particles with lower fluorescence than unstained target cells. Discrimination of dimly fluorescent target cells from the negative control cells proves that the instrument is sensitive enough to perform the immunophenotyping assay.
FORM
The Negative Control is prepared from chicken erythrocytes that have been processed to remove leukocytes. The fixed cells are suspended in Dulbecco's PBS and supplied in 2.0 mL volumes. The cell count per milliliter is at least 2.5 X 107 negative cells.
STABILITY
The Negative Control is stable at 2° - 8° C until the expiration date denoted on each vial label. Freshly diluted (1:10) preparations are stable for at least 12 hours when stored at 2° - 8° C.
PREPARATION OF WORKING SOLUTION
The recommended working solution is a 1:10 dilution of the stock solution in particulate-free physiological saline or isotonic buffered saline. This provides an adequate cell density and sufficient working solution for multiple assays.
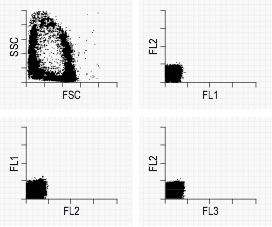
Typical Negative Control graphs
1. Thoroughly mix the Negative Control stock, and add one or two drops into a test tube (13 X 75 mm).
2. Add 0.9 mL of diluent, mix, and evaluate.
PRECAUTIONS
Although the Negative Control has been thoroughly washed to remove residual fixative, the chemical is hazardous, and it is recommended that gloves and eye protection be worn when handling this product.